 Tendons. When they’re injured, they hurt, and when they hurt, function can be significantly impaired.
Tendons. When they’re injured, they hurt, and when they hurt, function can be significantly impaired.
Treatment of tendon disorders constitutes a significant portion of my medical practice. While we still have much to learn about tendon physiology and repair mechanisms, recent research has provided better understanding of tendon disorders and offers new insights into potential treatment options.
In this month’s column we will consider the anatomy and physiology of tendons so that we can more completely understand available tendon treatments, which will be reviewed in the column to follow.
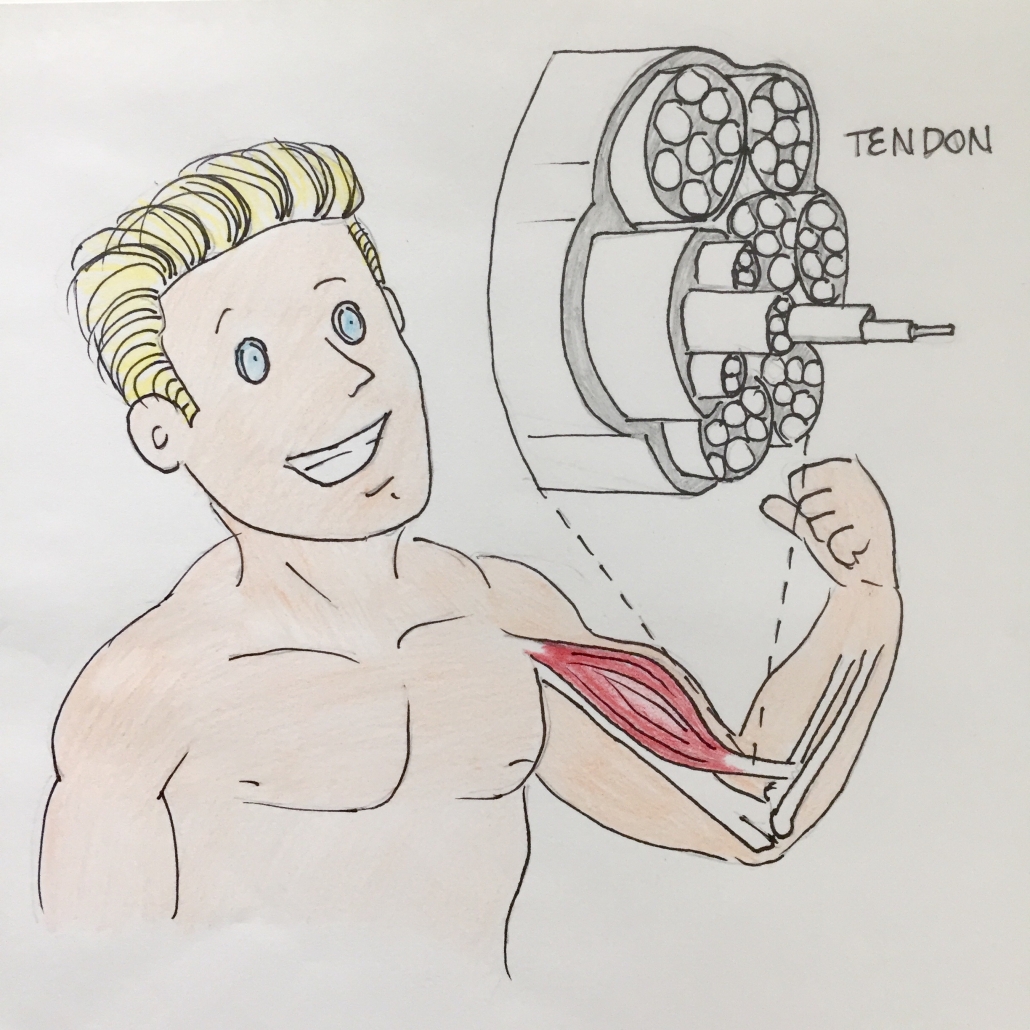
Tendons are robust bands of connective tissue that connect muscle to bone or muscle to muscle (whereas ligaments connect one bone to another). They’re comprised of densely packed collagen fibers, approximately 98% of which are type I collagen.
Tendons demonstrate a highly organized hierarchical structure which contributes to their high tensile strength. This can be likened to a woven wire rope.
In addition to collagen, tendons contain proteoglycans and glycoproteins – molecules that contribute to collagen fibril formation and to the mechanical properties of tendons.
Living within tendons are cells called tenocytes. These spindle-shaped cells, located along collagen fibers, are primarily responsible for maintaining tendon homeostasis by communicating with each other via channels known as gap junctions, and modifying tendon structure in response to various chemical and mechanical changes in the environment. This communication is often quite complex and involves a number of molecular signals and cellular receptors. When a tendon is placed under load, the tenocytes send signals to each other in order to encourage collagen formation and fortification of the tendon matrix. Some of these signals include transforming growth factor-β1 (TGF-β1), connective tissue growth factor, and interleuken-6 (IL-6).
Traditionally, tendons have been considered mere connections between muscles and bones, passively conducting forces during normal movement. Over the past few decades, however, a significant amount of research has focused on the dynamic and elastic properties of tendons and their ability to function as springs. Whereas some tendons predominantly function to position limbs (think of tendons in the fingers), others act as energy storing springs to make movement more efficient (think Achilles tendons). Interestingly, it has been shown that people with higher calves, and therefore longer Achilles tendons, are often better at running and jumping than those with shorter tendons since the longer tendon length contributes greater elastic recoil and more mechanically/energetically efficient movement. Tendon length is also a discerning factor in muscle size and shape, where individuals with shorter tendons generally have a greater potential for muscle mass.
While regular application of tension is essential for the fortification of tendon structures, excess and abnormal mechanical load can override the capacity of the tendons and associated tenocytes to maintain homeostasis, therefore contributing tendinopathy – disease of a tendon. Generally, tendon injuries can be categorized as chronic degenerative tendinopathies or acute ruptures. Even in the majority of acute ruptures, chronic degenerative changes are thought to precede rupture.
 When tendons become diseased, there is a loss of normal tendon architecture and often death of tenocytes. For some time it was believed that tendons are largely incapable of architectural and tenocyte repair. While this has since been shown to be incorrect, the natural healing process of tendons is rather slow, which is largely due to the relatively low cellularity and vascularity of tendon tissue.
When tendons become diseased, there is a loss of normal tendon architecture and often death of tenocytes. For some time it was believed that tendons are largely incapable of architectural and tenocyte repair. While this has since been shown to be incorrect, the natural healing process of tendons is rather slow, which is largely due to the relatively low cellularity and vascularity of tendon tissue.
The normal healing process occurs in three main stages: inflammation, proliferation, and remodeling. In the first stage, which typically occurs during the first week following an injury, inflammatory cells including neutrophils, monocytes, and lymphocytes migrate to the site of tendon damage where they begin to clean up dead tissue and cellular debris. Throughout approximately four weeks, a host of chemical signals are released, causing tenocytes to proliferate and move into the injured area in order to begin synthesizing type III collagen and other proteins. This is the second stage of healing. During the final phase, the tendon components are remodeled through collagen turnover, realignment, and formation of collagen cross-links in order to create a more organized and strong architecture.
While the natural healing process can repair tendon defects, studies have shown that the resultant tendon scar is often insufficient and the biomechanical properties of the new tissue seldom match pre-injury properties. This is likely the result of a number of factors including disorganized collagen alignment and thinner collagen fiber formation, which makes the healed tissue persistently vulnerable to future injury. Because of this, there has been much interest in treatments that may encourage more rapid and robust healing of tendon injuries. These will be reviewed in the upcoming article. Stay tuned!